Model introduction: Difference between revisions
No edit summary |
No edit summary |
||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 9: | Line 9: | ||
This interactions are illustrated below. Orientational modulations of the stacking potential encourage the bases to form coplanar stacks, the twist arising from the different length scales of the backbone separation and the optimum stacking separation. The possibility of unstacking allows single strands to be very flexible. Hydrogen bonding can occur between complementary bases when they are anti-aligned, leading to the formation of double helical structures. | This interactions are illustrated below. Orientational modulations of the stacking potential encourage the bases to form coplanar stacks, the twist arising from the different length scales of the backbone separation and the optimum stacking separation. The possibility of unstacking allows single strands to be very flexible. Hydrogen bonding can occur between complementary bases when they are anti-aligned, leading to the formation of double helical structures. | ||
[[File:the_model_half.png|500px]] | |||
(a) Model interaction sites with their interaction ranges (the typical range of an interaction is twice the radius of the sphere shown). | (a) Model interaction sites with their interaction ranges (the typical range of an interaction is twice the radius of the sphere shown). | ||
| Line 17: | Line 17: | ||
(c) A duplex in this representation. | (c) A duplex in this representation. | ||
[[File:interactions.png|600px]] | |||
Indication of the interactions which hold together a typical duplex. | |||
In the original model, all complementary base pairs and stacking partners interact with the same strength (there is no attractive interaction between non-complementary bases). A sequence-dependent parameterisation of the hydrogen-bonding and stacking interactions is included as an option in the code release. | |||
The melting temperatures of a set of short DNA oligomers in the sequence-dependent coarse-grained model, compared to the melting temperatures as predicted by SantaLucia's nearest-neighbor model ([http://www.pnas.org/content/95/4/1460.full]), are available here: [http://www-thphys.physics.ox.ac.uk/people/PetrSulc/data/CG_model_Tm.txt] | |||
The original model incorporates electrostatics only through the short-ranged excluded volume. For this reason, it is only appropriate for the study of systems at high salt concentration, when electrostatic interactions are strongly screened. It also does not incorporate the differentiation between the major and minor grooves of DNA double helices. | |||
===oxDNA2=== | |||
A new version of the oxDNA model, called oxDNA2, has been released in 2015 ([http://arxiv.org/abs/1504.00821],[http://scitation.aip.org/content/aip/journal/jcp/142/23/10.1063/1.4921957]). It introduces different widths for the major and minor DNA double helical grooves (an example double helix is shown below), a new electrostatic interaction which allows DNA to be studied at salt concentrations equivalent to 0.1M <nowiki>[NaCl]</nowiki> and above, improved large-scale structure prediction, and differentiation between AA and TT stacking strengths. | |||
https://dna.physics.ox.ac.uk/images/3/37/Perfect_yesmm_nopov.png | |||
The oxDNA2 model is included in the latest release of the oxDNA code. It can be used in a very similar way to the original oxDNA model. Only a couple of lines in the input file must be added. Firstly, the following line must be included: | |||
<pre> | |||
interaction_type = DNA2 | |||
</pre> | |||
In addition, the salt concentration for the simulation must be specified. For example, to run a simulation with a salt concentration of 0.5M, one should write: | |||
<pre> | |||
salt_concentration = 0.5 | |||
</pre> | |||
Everything else should work in the same way as it does for the original oxDNA model. | |||
===Simulation units=== | ===Simulation units=== | ||
The code uses | The code uses units for energy, mass, length and time that are convenient for a typical system. The relationship between simulation units (SU) and SI units is given below. | ||
{| | {| | ||
| Line 36: | Line 55: | ||
|- | |- | ||
| 1 unit of length | | 1 unit of length | ||
| 8.518x10<math>^{-10}</math> m | | 8.518x10<math>^{-10}</math> m = 0.8518 nm | ||
|- | |- | ||
| 1 unit of energy | | 1 unit of energy | ||
| 4. | | thermal energy at 3000K - approximately 4.142x10<math>^{-20}</math> J = 41.42 pN nm | ||
|- | |- | ||
| 1 unit of | | 1 unit of temperature | ||
| 3000 K | | 3000 K | ||
|- | |- | ||
| 1 unit of | | 1 unit of force | ||
| 4. | | 4.863x10<math>^{-11}</math> N = 48.63 pN | ||
|- | |||
| 1 unit of mass | |||
| 5.24x10<math>^{-25}</math> kg | |||
|- | |||
| 1 unit of time | |||
| 3.03x10<math>^{-12}</math> s = 3.03 ps | |||
|- | |- | ||
| 1 unit of | | 1 unit of force constant (1 unit force/1 unit length) | ||
| | | 57.09 pN/nm | ||
|- | |- | ||
| 1 unit of | | 1 unit of torque (1 unit force * 1 unit length) | ||
| | | 41.423 pN * nM | ||
|} | |||
===Useful conversions=== | |||
{| | |||
|- | |- | ||
| Boltzmann's constant - in simulation units, we set Boltzmann's constant to 1 so that we can measure temperature and energy with the same unit. Therefore in simulation units energy/K Boltzmann's constant is 0.001/3. | |||
|} | |} | ||
| Line 64: | Line 95: | ||
[http://link.aip.org/link/?JCP/134/085101 Structural, mechanical and thermodynamic properties of a coarse-grained DNA model] ([http://arxiv.org/abs/arXiv:1009.4480 arXiv]) | [http://link.aip.org/link/?JCP/134/085101 Structural, mechanical and thermodynamic properties of a coarse-grained DNA model] ([http://arxiv.org/abs/arXiv:1009.4480 arXiv]) | ||
P. Šulc, F. Romano, T. E. Ouldridge, L. Rovigatti, J. P. K. Doye, A. A. Louis, | P. Šulc, F. Romano, T. E. Ouldridge, L. Rovigatti, J. P. K. Doye, A. A. Louis, ''J. Chem. Phys.'' '''137''', 135101 (2012) | ||
[http:// | [http://jcp.aip.org/resource/1/jcpsa6/v137/i13/p135101_s1 Sequence-dependent thermodynamics of a coarse-grained DNA model] ([http://arxiv.org/abs/1207.3391 arxiv]) | ||
B. E. K. Snodin, F. Randisi, M. Mosayebi, P. Šulc, J. S. Schreck, F. Romano, T. E. Ouldridge, R. Tsukanov, E. Nir, A. A. Louis, J. P. K. Doye, ''J. Chem. Phys.'' '''142''', 234901 (2015) | |||
[http://scitation.aip.org/content/aip/journal/jcp/142/23/10.1063/1.4921957 Introducing Improved Structural Properties and Salt Dependence into a Coarse-Grained Model of DNA] ([http://arxiv.org/abs/1504.00821 arXiv]) | |||
Latest revision as of 15:34, 13 May 2020
The model treats DNA as a string of rigid nucleotides, which interact through potentials which depend on the position and orientation of the nucleotides. The interactions are:
- Sugar-phosphate backbone connectivity,
- Excluded volume,
- Hydrogen bonding,
- Nearest-neighbour stacking,
- Cross-stacking between base-pair steps in a duplex,
- Coaxial stacking.
This interactions are illustrated below. Orientational modulations of the stacking potential encourage the bases to form coplanar stacks, the twist arising from the different length scales of the backbone separation and the optimum stacking separation. The possibility of unstacking allows single strands to be very flexible. Hydrogen bonding can occur between complementary bases when they are anti-aligned, leading to the formation of double helical structures.
(a) Model interaction sites with their interaction ranges (the typical range of an interaction is twice the radius of the sphere shown).
(b) Representation of these interaction site in a visualisation that makes the planarity of the base clear.
(c) A duplex in this representation.
Indication of the interactions which hold together a typical duplex.
In the original model, all complementary base pairs and stacking partners interact with the same strength (there is no attractive interaction between non-complementary bases). A sequence-dependent parameterisation of the hydrogen-bonding and stacking interactions is included as an option in the code release. The melting temperatures of a set of short DNA oligomers in the sequence-dependent coarse-grained model, compared to the melting temperatures as predicted by SantaLucia's nearest-neighbor model ([1]), are available here: [2]
The original model incorporates electrostatics only through the short-ranged excluded volume. For this reason, it is only appropriate for the study of systems at high salt concentration, when electrostatic interactions are strongly screened. It also does not incorporate the differentiation between the major and minor grooves of DNA double helices.
oxDNA2
A new version of the oxDNA model, called oxDNA2, has been released in 2015 ([3],[4]). It introduces different widths for the major and minor DNA double helical grooves (an example double helix is shown below), a new electrostatic interaction which allows DNA to be studied at salt concentrations equivalent to 0.1M [NaCl] and above, improved large-scale structure prediction, and differentiation between AA and TT stacking strengths.
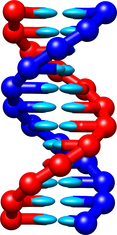
The oxDNA2 model is included in the latest release of the oxDNA code. It can be used in a very similar way to the original oxDNA model. Only a couple of lines in the input file must be added. Firstly, the following line must be included:
interaction_type = DNA2
In addition, the salt concentration for the simulation must be specified. For example, to run a simulation with a salt concentration of 0.5M, one should write:
salt_concentration = 0.5
Everything else should work in the same way as it does for the original oxDNA model.
Simulation units
The code uses units for energy, mass, length and time that are convenient for a typical system. The relationship between simulation units (SU) and SI units is given below.
| Simulation unit | Physical unit |
|---|---|
| 1 unit of length | 8.518x10 m = 0.8518 nm |
| 1 unit of energy | thermal energy at 3000K - approximately 4.142x10 J = 41.42 pN nm |
| 1 unit of temperature | 3000 K |
| 1 unit of force | 4.863x10 N = 48.63 pN |
| 1 unit of mass | 5.24x10 kg |
| 1 unit of time | 3.03x10 s = 3.03 ps |
| 1 unit of force constant (1 unit force/1 unit length) | 57.09 pN/nm |
| 1 unit of torque (1 unit force * 1 unit length) | 41.423 pN * nM |
Useful conversions
| Boltzmann's constant - in simulation units, we set Boltzmann's constant to 1 so that we can measure temperature and energy with the same unit. Therefore in simulation units energy/K Boltzmann's constant is 0.001/3. |
The model and its performance is discussed in detail in the following references (the thesis provides the most complete analysis):
T. E. Ouldridge, D.Phil. Thesis, University of Oxford, 2011. Coarse-grained modelling of DNA and DNA self-assembly
T. E. Ouldridge, A. A. Louis and J. P. K. Doye, J. Chem. Phys, 134, 085101 (2011) Structural, mechanical and thermodynamic properties of a coarse-grained DNA model (arXiv)
P. Šulc, F. Romano, T. E. Ouldridge, L. Rovigatti, J. P. K. Doye, A. A. Louis, J. Chem. Phys. 137, 135101 (2012) Sequence-dependent thermodynamics of a coarse-grained DNA model (arxiv)
B. E. K. Snodin, F. Randisi, M. Mosayebi, P. Šulc, J. S. Schreck, F. Romano, T. E. Ouldridge, R. Tsukanov, E. Nir, A. A. Louis, J. P. K. Doye, J. Chem. Phys. 142, 234901 (2015) Introducing Improved Structural Properties and Salt Dependence into a Coarse-Grained Model of DNA (arXiv)






